Book Nook Cafe discussion
Book Buddy !
>
The Disappearing Spoon ~ March 2013
 Discussion questions to consider while reading the book.
Discussion questions to consider while reading the book.1. Which story about the periodic table or the elements stood out most for you?
2. Sam Kean's writing style is unique. What did you think about how this book was written?
3. Dmitri Mendeleev is usually associated with the periodic table. Were you surprised to learn about the other scientists that played a role in its development?
4. Did you read the notes for each chapter? Would you have preferred this information to be included as footnotes, rather than at the end of the book?
5. Did you like how Kean personified the elements? Were there any characterizations with which you disagreed?
6. Did you feel that the science in this book was presented well for a layperson to understand? Were there any concepts that are still fuzzy to you?
7. Each chapter of this book weaves history, science, and sometimes art and literature, to tell the story of the elements of the periodic table. Did you sometimes feel there was too much information to absorb?
8. After reading The Disappearing Spoon, do you agree with Kean as he asserts in the introduction of the book that the periodic table is "the history of our species written in a compact and elegant script" (p.8)?
the above questions are from:
http://www-library.ncifcrf.gov/bookcl...
Did the book engage you ? Why or why not?
Did it take awhile to get into it or were you immediate into it.
What passage struck you as insightful, even profound?
If you could ask the author a question, what would you ask?
Has the book changed you? Broadened your perspective? Have you learned something new?
What was the central idea of the book?
Talk about specific passages that struck you as significant - or interesting, profound, amusing, illuminating, disturbing, sad..? What was memorable?
What have you learned after reading this book? Has it broadened your perspective?
 I just downloaded this to my Nook--I found it on sale at Barnes & Noble today for 20% off, which made it $7.99. The paperback is 31% off today, so the regular $14.99 price is now $10.29. I don't know if it's a one-day-only sale or not, but if anyone is interested, that's the price for today, at least!
I just downloaded this to my Nook--I found it on sale at Barnes & Noble today for 20% off, which made it $7.99. The paperback is 31% off today, so the regular $14.99 price is now $10.29. I don't know if it's a one-day-only sale or not, but if anyone is interested, that's the price for today, at least!
 I would like to join you in reading this book too. I always found Chemistry fascinating in high school and college.
I would like to join you in reading this book too. I always found Chemistry fascinating in high school and college.
 I was too much of a dunce in high school to enroll in Chemistry but i have always been intrigued by the periodic table. I'm not sure how odd that is or not. Maybe it's the idea of organizing all the info so compactly? I really hope i get to join the group in exploring this book but, if not, when i do i'll know the posts & thread will still be available to me.
I was too much of a dunce in high school to enroll in Chemistry but i have always been intrigued by the periodic table. I'm not sure how odd that is or not. Maybe it's the idea of organizing all the info so compactly? I really hope i get to join the group in exploring this book but, if not, when i do i'll know the posts & thread will still be available to me.
 This book is REALLY fun! It's mostly stories and fun tidbits so those not science-ly oriented will have no problem, and those who are will garner a few more fun facts not learned before. Puzzlingly, in the earliest part of the book (first chapter of so) there are a couple of explanations and/or graphs he could have easily included to make things more clear for the uninitiated. Otherwise, the book is just a lot of fun and very entertaining.
This book is REALLY fun! It's mostly stories and fun tidbits so those not science-ly oriented will have no problem, and those who are will garner a few more fun facts not learned before. Puzzlingly, in the earliest part of the book (first chapter of so) there are a couple of explanations and/or graphs he could have easily included to make things more clear for the uninitiated. Otherwise, the book is just a lot of fun and very entertaining.BTW, for those with iPads, you can dl an app which shows the periodic table. I used to have a poster of the table on my wall (I'm not a chemist), but now can just go to my iPad when I have a question about something. I'm sure there are also many online versions of the periodic table, as well as apps for other devices which have apps.
 Thanks for that tip, Libyrinths. I recall when you read it that you mentioned the lack of what seems to be a fundamental graphic. I first studied the table in my dictionary, which was cool. However, these old eyes can't see most of the printing nowadays. :-)
Thanks for that tip, Libyrinths. I recall when you read it that you mentioned the lack of what seems to be a fundamental graphic. I first studied the table in my dictionary, which was cool. However, these old eyes can't see most of the printing nowadays. :-)
 Oh, just in time! Been reading this only every now and then because I usually have to check things up in the periodic table. Having book buddies for this one will surely inspire me to pick up where I left off :)
Oh, just in time! Been reading this only every now and then because I usually have to check things up in the periodic table. Having book buddies for this one will surely inspire me to pick up where I left off :)
 Alias Reader wrote: "Welcome to Book Nook Cafe, Katrina ! I'm so glad you will be joining us for this read."
Alias Reader wrote: "Welcome to Book Nook Cafe, Katrina ! I'm so glad you will be joining us for this read."Thank you! So glad to be welcome here!
 There are 25 copies statewide which are out. Hopefully it's a book group who will read / discuss it this month, and will be returning it soon!
There are 25 copies statewide which are out. Hopefully it's a book group who will read / discuss it this month, and will be returning it soon!
 Thanks for going the extra mile, Connie.
Thanks for going the extra mile, Connie. I forgot to write down who the original posters were who wanted this Buddy Read. If they want to take the lead in this discussion that is fine by me. If not, that's cool, too.
I know very little about science and haven't thought of the periodic table since I was a kid in school. So I hope I can contribute something to the conversation. But I am game to try ! :)
I have a request in for the book at my library. I hope to get it my March 1st and begin reading it then.
 Feb. 25
Feb. 25I don't know if she is mentioned in the book, but I thought I would post the birthday of Ida Noddack.
Ida Noddack ~~25 February 1896 - 29 October 1978
One of the first prominent female chemists in Germany, Noddack was nominated several times for a Nobel Prize but never won. Still, she made a number of remarkable contributions to science, co-discovering the element rhenium with her future husband and proposing for the first time the idea of nuclear fission. She also participated in the discovery of another element, which her team named masurium, but they could not prove its existence. It was finally isolated by others in 1937 and named rhenium. She was nominated three times for Nobel Prize in Chemistry.
http://en.wikipedia.org/wiki/Ida_Noddack

 I just started reading this today (only on the first chapter)--are we supposed to be up to a certain point by Friday, or are we just free-forming it as we all go?
I just started reading this today (only on the first chapter)--are we supposed to be up to a certain point by Friday, or are we just free-forming it as we all go?
 Free form.
Free form. As with all our group reads we do ask:
Spoiler Etiquette: The book is divided up into 5 parts. PLEASE put the PART & Chapter # at the top of your post.
However, with this book I don't think spoilers come into play. However putting the Part & or Chapter # will help people follow along with you.
I picked up the book today from the library. I hope to start it tomorrow or the next day. What I know about science is nil, so I hope I can keep up with you all !
 First, if anyone knows anything at all about chemistry and would like to lead the book discussion, please do.
First, if anyone knows anything at all about chemistry and would like to lead the book discussion, please do.What I know about chemistry is zero. It's been a hundred years since I took it in junior high school.
Second, in an attempt to understand the book, I am going to post some links and definitions. If you have any please don't hesitate to share.
Element:
An element is a substance made up of only one type of atom.
What is an element? How many elements are there?
An element is a substance that is made entirely from one type of atom. For example, the element hydrogen is made from atoms containing a single proton and a single electron. If you change the number of protons an atom has, you change the type of element it is.
If you had very, very good eyes and could look at the atoms in a sample of hydrogen, you would notice that most of the hydrogen atoms would have no neutrons, some of them would have one neutron and a few of them would have two neutrons. These different versions of hydrogen are called isotopes. All isotopes of a particular element have the same number of protons, but have a different number of neutrons. If you change the number of neutrons an atom has, you make an isotope of that element.
As of October 16, 2006, scientists know of 117 different elements. Some, like gold, silver, copper and carbon, have been known for thousands of years. Others, such as meitnerium, darmstadtium and ununquadium, have only recently been created by scientists. All known elements are arranged on a chart called the Periodic Table of Elements.
Atom:
What is an atom? What are atoms made of?
Atoms are the basic building blocks of ordinary matter. Atoms can join together to form molecules, which in turn form most of the objects around you.
Atoms are composed of particles called protons, electrons and neutrons. Protons carry a positive electrical charge, electrons carry a negative electrical charge and neutrons carry no electrical charge at all. The protons and neutrons cluster together in the central part of the atom, called the nucleus, and the electrons 'orbit' the nucleus. A particular atom will have the same number of protons and electrons and most atoms have at least as many neutrons as protons.
Protons and neutrons are both composed of other particles called quarks and gluons. Protons contain two 'up' quarks and one 'down' quark while neutrons contain one 'up' quark and two 'down' quarks. The gluons are responsible for binding the quarks to one another.
Periodic table:
The periodic table is a chart containing information about the atoms that make up all matter.
A periodic table is a tabular display of the chemical elements, organized on the basis of their atomic numbers, electron configurations, and recurring chemical properties.
The periodic table is a table of the chemical elements, showing the symbols for the elements, their full names, their atomic numbers and their mass number. It organizes them into groups and periods (columns and rows) based on their structure and properties. It is called "the periodic table" because the horizontal rows are named "periods."
A good link to the table. Better than the one in my hardcover book, anyway. Put your cursor over the element to get a larger picture.
http://www.ptable.com/
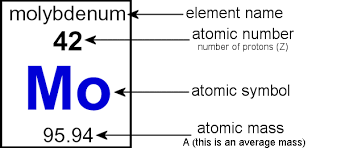
Atomic number

The atomic number is equal to the number of protons in an atom's nucleus. The atomic number determines which element an atom is. For example, any atom that contains exactly 47 protons in its nucleus is an atom of silver.
Atom:
What is an atom?
An atom a fundamental piece of matter. (Matter is anything that can be touched physically.) Everything in the universe (except energy) is made of matter, and, so, everything in the universe is made of atoms.
An atom itself is made up of three tiny kinds of particles called subatomic particles: protons, neutrons, and electrons. The protons and the neutrons make up the center of the atom called the nucleus and the electrons fly around above the nucleus in a small cloud. The electrons carry a negative charge and the protons carry a positive charge. In a normal (neutral) atom the number of protons and the number of electrons are equal. Often, but not always, the number of neutrons is the same, too.
Atomic Mass:
: Atomic mass or atomic weight is the average mass of atoms of an element, calculated using the relative abundance of isotopes in a naturally-occurring element.
The mass of an atom expressed in atomic mass units. It is approximately equivalent to the number of protons and neutrons in the atom...
*** Youtube video explaining the atom- good refresher
https://www.khanacademy.org/science/c...
Here is a YouTube explaining the table structure.
http://www.youtube.com/watch?v=6wDJYN...
 The element song
The element songhttp://www.youtube.com/watch?v=DYW50F...
For you Big Bang Theory Fans, here is Sheldon singing the song. :)
http://www.youtube.com/watch?v=T6WDJ1...
Here is Parsons commenting on the song on Conan
http://www.youtube.com/watch?v=V0CeO9...
Too difficult you say...Watch this 5 year old.
http://www.youtube.com/watch?v=p5pU7I...
 RE: Intro to book.
RE: Intro to book.I like the sentiment expressed by the author explaining how though the periodic table he also
"...learned history, etymology, alchemy, mythology, literature, poison forensics, and psychology."
 Re: Intro to book
Re: Intro to bookHere is Dr. Benjamin Rush of the famous Bilious Pills.

http://en.wikipedia.org/wiki/Benjamin...

Here is a link to the label on the pills
http://lewis-clark.org/content/conten...
 Dmitri Mendeleev
Dmitri MendeleevDmitri Ivanovich Mendeleev was a Russian chemist and inventor. He created the first version of the periodic table of elements, and used it to predict the properties of elements yet to be discovered. Wikipedia
Born: February 8, 1834, Tobolsk
Died: February 2, 1907, Saint Petersburg
Nationality: Russian
Awards: Copley Medal, Demidov Prize
Education: Ruprecht Karl University of Heidelberg, Saint Petersburg State University


 I don't know enough about chemistry to lead a discussion, either. I thought I would just post my thoughts as I go along.
I don't know enough about chemistry to lead a discussion, either. I thought I would just post my thoughts as I go along.INTRODUCTION:
1. Liked the comment about how heavily ancient languages and mythology influenced the periodic table, with the example of the symbol for mercury (Hg) being derived from the Latin word "hydragyrum" or "water silver."
2. In response to your comment, Alias, I also highlighted this sentence: "Between hydrogen at the top left and the manmade impossibilities lurking along the bottom, you can find bubbles, bombs, money, alchemy, petty politics, history, poison, crime, and love. Even some science." That made me LOL.
 Amy wrote: 1. Liked the comment about how heavily ancient languages and mytholody influenced the periodic table, with the example of the symbol for mercury (Hg) being derived from the Latin word "hydragyrum" or "water silver."
Amy wrote: 1. Liked the comment about how heavily ancient languages and mytholody influenced the periodic table, with the example of the symbol for mercury (Hg) being derived from the Latin word "hydragyrum" or "water silver."--------------------
Amy, my hardcover has the Periodic Table in the back. However, it just gives the symbol, not the name. I wouldn't have done that if I was the author. But what do I know. ANYway, I was looking all over for something with a "M" for mercury. I ended up looking in my dictionary and seeing that it is HG80. The author does say this later on.
 Chapter 1
Chapter 1Reading about Maria Goeppert-Mayer really brings home how much things have changed for women since the 1930s, although it's still much harder for women to break into some areas of science. Because of sexism and strict rules against nepotism, she was working as a volunteer at a university where her husband held a position. She won a Nobel prize in Physics for her work regarding the nuclear shell structure.
http://en.wikipedia.org/wiki/Maria_Go...
http://www.nobelprize.org/nobel_prize...
 Lori wrote: "Just got my book. Let the reading begin!"
Lori wrote: "Just got my book. Let the reading begin!"--------------
:)
I am going to try and get some reading in this evening.
 Connie wrote: "Chapter 1
Connie wrote: "Chapter 1Reading about Maria Goeppert-Mayer really brings home how much things have changed for women since the 1930s, although it's still much harder for women to break into some areas of scienc..."
--------------------------
Thanks for the links, Connie. I am going to try and read chapter 1 this evening.
Regarding your comment on women. At an author meeting I attended for another book, the author commented that during the 20's 30s and 40s women were much freer than the 1950s on.
He mention Emelia Earhart for example. She wore pants and flew an airplane ! During the war women worked. They got the right to vote in the 1920s.
He mentioned the recent book on Prohibition. I think he said it became more acceptable after Prohibition to see women drinking.
The delineation between that period and the 50s to 1960 is interesting. Anyone know a good book on this topic?
I have a feeling Disappearing Spoon is going to increase my TBR ! :)
 Alias Reader wrote: "He mentioned the recent book on Prohibition. I think he said it became more acceptable after Prohibition to see women drinking..."
Alias Reader wrote: "He mentioned the recent book on Prohibition. I think he said it became more acceptable after Prohibition to see women drinking..."My husband just finished reading Prohibition: Thirteen Years That Changed America by Edward Samuel Behr, and was really engrossed in it. He said it was really good--if you are looking for another one to add to your TBR. ;)
 Chapter 2:
Chapter 2:1) As a word person, I appreciate the author's explanation of how to use a knowledge of etymology and language to parse the prefixes and suffixes of chemical compounds into understandable parts.
2) I also appreciate how he compares the periodic table to genealogy, which makes things much more clear in my mind, for some reason!
3) The discussion about germanium vs. silicon was fascinating. Although William Shockley sounds like a ruthless jerk.Especially the part about how he eventually abandoned solid-state physics to pursue the "science" of eugenics. Happy to see that Bardeen received recognition from the Nobel committee--even if he was chastized for making his sons stay in school instead of traveling to the award ceremony with him!
4) Another interesting story of Jack Kilby and how he invented integrated circuits because he was a new employee and didn't have vacation time during the summer! Little details like these are the most interesting part of the book for me.
 Connie wrote: "Chapter 1
Connie wrote: "Chapter 1Reading about Maria Goeppert-Mayer really brings home how much things have changed for women since the 1930s, although it's still much harder for women to break into some areas of scienc..."
---------------
I was shocked that the universities could get away with out paying her !
 Chapter 3:
Chapter 3:1) Was immediately intrigued by the discussion of arsenic--how Roman assassins used to smear it on figs, and how the best antidote for arsenic poison (used to this day) was developed by none other than Robert Bunsen, otherwise known to high school students everywhere as the guy who perfected that burner on the lab counter in chemistry class.
2) I was unaware that Mendeleev was not the first person to come up with the periodic table, and that others had been working independently on the organizational project. I was struck by this line: "Science needs heroes as much as any other field, and Mendeleev became the protagonist of the story of the periodic table for a number of reason..." Partly because his back story was so arresting, but mostly because, as the author put it, he "had balls enough to predict that new elements would be dug up." Being able to predict the densities and atomic weights of elements that weren't even discovered yet must have made Mendeleev seem almost like a seer! And I laughed out loud at the comment made by the tsar when complaints were lodged against Mendeleev's bigamy: "I admit, Mendeleev has two wives, but I only have one Mendeleev."
3) I highlighted this line about the discovery of gallium, which struck me while I was reading: "It raised the question of what really drives science forward--theories, which frame how people view the world, or experiments, the simplest of which can destroy elegant theories." It's sort of like which came first--the chicken or the egg? Does a theory tune a scientist into seeing something new that he decides to test? Or do experiments provide the evidence that frames a theory? Interesting to ponder, with Einstein's comment providing food for thought: "It is theory that decides what we can observe."
4) Loved the story that explains the title of "the disappearing spoon"!
5) Also enjoyed story of the village of Ytterby, Sweden, and its contribution to the periodic table. I always wondered where "yttrium" and "ytterbium" came from!
 Amy wrote: "Chapter 2:
Amy wrote: "Chapter 2:3) The discussion about germanium vs. silicon was fascinating. Although William Shockley sounds like a ruthless jerk.Especially the part about how he eventually abandoned solid-state physics to pursue the "science" of eugenics.
---------------
Yes, that was interesting. The electron/protons stuff I don't really get, but when he connects it to everyday stuff and people it really is fascinating.
Here is a pic of Shockley

William Bradford Shockley Jr. was an American physicist and inventor. Along with John Bardeen and Walter Houser Brattain, Shockley co-invented the transistor, for which all three were awarded the 1956 Nobel Prize in Physics. Wikipedia
Born: February 13, 1910, London
Died: August 12, 1989, Stanford
Education: Massachusetts Institute of Technology, California Institute of Technology
Books: Electrons and Holes in Semiconductors with Applications to Transistor Electronics
Awards: Nobel Prize in Physics, IEEE Medal of Honor
http://en.wikipedia.org/wiki/William_...
Interesting stuff about transistors. Funny I was just talking to someone about how kids probably don't know what a transistor radio is.
 I took college level chemistry in high school (many, many years ago) but I won't be starting the book until closer to the end of the week. I might be able to help with questions though. Those definitions you posted Alias, are a pretty good, simple explanation. Yes, not all elements letters match up alphabetically with their name...mercury is HG, gold is AU. I think maybe it is from the latin names?
I took college level chemistry in high school (many, many years ago) but I won't be starting the book until closer to the end of the week. I might be able to help with questions though. Those definitions you posted Alias, are a pretty good, simple explanation. Yes, not all elements letters match up alphabetically with their name...mercury is HG, gold is AU. I think maybe it is from the latin names?
 Julie wrote: "I took college level chemistry in high school (many, many years ago) but I won't be starting the book until closer to the end of the week. I might be able to help with questions though. Those defin..."
Julie wrote: "I took college level chemistry in high school (many, many years ago) but I won't be starting the book until closer to the end of the week. I might be able to help with questions though. Those defin..."Yes, they are from the Latin and the Greek, a lot of them. That's one advantage I have when reading this--it's not the chemistry I took in college, it's the 6 years of Latin in high school and college! I can almost always figure out what the symbol is if I can remember the Latin words. It's been so long--I'm sort of surprised that the Latin is coming back to me so easily. I guess things that are buried deep down in the brain can still be accessed (too many) years later!
 Part 1 of 5
Part 1 of 5I see from a google search that I was wrong in my guess of what ETA before an element means. I thought maybe "element know as". Which didn't make much sense.
Google: ETA
a prefix used to designate the first element of the same family in the periodic table beyond the one to whose name it is prefixed, as ekaselenium for technetium.
Though I am still unclear what the author means when he write on p.54 hardcover edition-
"The discovery of eka-aluminium, now known as gallium..."
In the column aluminium13 is above gallium31. What does he mean replaced by gallium?
Here again is a link to the periodic table.
http://www.webelements.com/
If I learned about the spectroscope in school it is long forgotten. I think it's pretty cool that elements can be identified by unique bands of color when heated.
Here is an image of a spectroscope.

I thought the section on porcelain was interesting. Here is the image of the Porcelain Tower of Nanjing.

http://en.wikipedia.org/wiki/Porcelai...
Though it's kind of annoying that the author just writes, "Another claimed the Chinese were fabulously wealthy in porcelain that they had erected a nine-story tower of it, just to show off. (that one turned out to be true.)"
Would it have killed the author to name the tower? Thank heaven Google is not so stingy as this author is with definitions and other info.
Speaking of the authors writing, at the close of Part 1, I have some issues with it.
One, a good glossary would be nice.
Two, he has an irreverent way of writing that I think is supposed to be hip/cool. I don't know it sort of leave me cold. For example he writes in chapter 3, "...Mendeleev, unlike the squeamish Meyer, had balls enough to predict that new elements would be dug up."
Lastly, a good periodic color table with the names of the elements would seem to be a must in a book devoted to the subject. Instead in my hardcover edition, in the back, is a very uninspiring periodic table, sans color and the names of the elements.
 More on "eka":
More on "eka":On page 53 of the hardcover book, the author says that eka is the Sanskrit word for "beyond".
The scientists are using that terminology before the element was actually found, and was just an element that they theorized should be there. When they found the actual element, it was given its new name. (Elements in the same vertical line in the periodic table act in similiar ways in chemical reactions.)
I think the beginning of the book is the hardest part for someone who has not taken a chemistry class to understand. So hang in there, Alias.
Chapter 5 deals with chemistry used to produce military weapons during World Wars I and II. It ties in with some of the World War II books we have read with this group. It's very sad when scientific discoveries are used for destruction instead of for the common good. I found it especially interesting how they made iron stronger and more heat resistant by adding other elements.
 Alias Reader wrote: "Two, he has an irreverent way of writing that I think is supposed to be hip/cool. I don't know it sort of leave me cold. For example he writes in chapter 3, "...Mendeleev, unlike the squeamish Meyer, had balls enough to predict that new elements would be dug up..."
Alias Reader wrote: "Two, he has an irreverent way of writing that I think is supposed to be hip/cool. I don't know it sort of leave me cold. For example he writes in chapter 3, "...Mendeleev, unlike the squeamish Meyer, had balls enough to predict that new elements would be dug up..." I actually like the writing style. It's making the subject material way more interesting to me. Every time he makes a comment like that one about Mendeleev, it makes me laugh out loud. I think it's very difficult to be able to write about hard science in a way that makes it accessible to those who are not scientists. Too many other books bog down with dry descriptions and details and attempts to explain the material in purely scientific ways. I'm enjoying the book a lot more because of the writing style.
 ETA
ETAConnie wrote:
The scientists are using that terminology before the element was actually found, and was just an element that they theorized should be there. When they found the actual element, it was given its new name. (Elements in the same vertical line in the periodic table act in similiar ways in chemical reactions.
-----------------
That is what I thought it meant. Sort of like a placeholder. However, the definition seems to be saying something different. If I am understanding it correctly it's the first in a series or family.
--a prefix used to designate the first element of the same family in the periodic table beyond the one to whose name it is prefixed, as ekaselenium for technetium.
 Part 2 Chapter 4
Part 2 Chapter 4Interesting chapter. I wish I had a better memory. This book would be excellent for Jeopardy trivia. ;)
It really is hard to wrap my mind about some of the things the author talks about. For example, Jupiter has had a hurricane 3x wider than earth that hasn't dissipated after centuries of storming ! It's just amazing and awe inspiring. I guess science can be like that. Mind boggling. :)
Cool Trivia- Pb Latin for lead and derives the word plumber.
I find the extinction of the dino's very interesting. I enjoyed reading the various theories. It's also interesting to remember that not just the dinosaurs died at that point but 75%of all species and 99% of all living beings. I knew this, but to read it still is fascinating.
I've read in other places about the extinction every 26 million years or so. I think I've read we are due. I hope no one comes to collect any time soon. :)
Here is the Wiki on this topic.
http://en.wikipedia.org/wiki/Extincti...
Here is the wiki on Alpha Centauri. -Nearest know star
http://en.wikipedia.org/wiki/Alpha_Ce...
Cool factoid- "All the elements other than hydrogen and helium make up just .04% of the universe."
~ Eric Scerri
http://www.chem.ucla.edu/dept/Faculty...
 The overall "star theme" of chapter 4 and the next few ones brought to mind a favorite poem by Walt Whitman:
The overall "star theme" of chapter 4 and the next few ones brought to mind a favorite poem by Walt Whitman:"When I heard the learn’d astronomer,
When the proofs, the figures, were ranged in columns before me,
When I was shown the charts and diagrams, to add, divide, and measure them,
When I sitting heard the astronomer where he lectured with much applause in the lecture-room,
How soon unaccountable I became tired and sick,
Till rising and gliding out I wander’d off by myself,
In the mystical moist night-air, and from time to time,
Look’d up in perfect silence at the stars."
 Chapter 4
Chapter 41) I was fascinated by the discussion about how Jupiter is "not a large planet so much as a failed star," and that if things had gone slightly different during its formation, our solar system would have been a binary system with two stars.
2) Interesting story about Clair Patterson's crusade against lead contamination, which resulted from his work in the lab and led to the ban on lead paint and leaded gasoline.
3) I also found the discussion of the various extinction theories fascinating, particularly the one that geologists believe massive volcanoes erupting in India right before and after the massive meteor impact in the Yucatan helped kill off the dinosaurs. I always thought it was just the meteor.
4) Given the fact that we recently had a "near-miss" with an asteroid, I think I'm reassured by the assertion that "an asteroid passing close to the sun has only slightly better than one chance in a billion of hitting our planet." I THINK. Although the subsequent comment that "we're on one long, deadly carousel ride through the universe" didn't make me feel very safe!
5) Love, love, LOVE that the chapter ended with a quote from Carl Sagan. When I was growing up, I was obsessed with Cosmos (both the book and the TV series).
 Chapter 5
Chapter 5I found this chapter incredibly interesting, despite the grim subject matter of chemical warfare. I learned so much.
1) Sad story of Fritz Haber and the path he took to develop gas weaponry for the Germans. Especially the story of his wife, who was a chemist herself and who disagreed with her husband's projects. I did find it rather ironic that he won the Nobel Prize in 1918 for his scientific body of work, and yet was charged as a war criminal in 1919 for that very same work. Also sadly ironic that the weapons he developed were eventually used to gas Haber's Jewish relatives in WWII. Karma is a real bitch.
2) I didn't know anything about the relationship between cell phones and the violence/anarchy in the Congo. Wow.
 Chapter 6
Chapter 61) More stuff I didn't know: That scientists developed new fields that expressly enable them to create new elements. I never really thought about the fact that the periodic table is bigger now than it was when I learned chemistry in high school. I guess I naively assumed that scientists just discovered new elements as they went along--I never imagined they were actually CREATING them in the lab!
2) Sad to read about the untimely death of Charles Moseley in the Gallipoli campaign of 1915. It seems like he was a man ahead of his time when it came to nuclear science. Wonder what he might have contributed to science had he lived past 27.
3) I read the discussion of the Manhattan Project with great interest, particularly the story of the women who were hired to crunch long tables of data. I was also particularly struck by this sentence, "after the project ended, scientists scattered back to their homes to reflect on what they'd done (some proudly, some not)." At the university where I work, we have a professor emeritus who worked on the Manhattan Project as a chemist. He was so disturbed by the work that he did on that project that when he left and came to teach at our college, he was instrumental in the creation of a new major: Peace and human rights. He spent the rest of his professional life teaching courses about the practice of peace and human rights from a broadly multicultural perspective.
4) Another interesting factoid that I learned: Hiroshima and Nagasaki were "more or less inhabitable" within days of the 1945 explosions. Days! I would not have guessed it was that quickly.
 Amy wrote: In the mystical moist night-air, and from time to time,
Amy wrote: In the mystical moist night-air, and from time to time,Look’d up in perfect silence at the stars."
------------------
:) Yes, sometimes we just want to enjoy the beauty and magic of the night sky.
 Amy wrote: "Chapter 4
Amy wrote: "Chapter 42) Interesting story about Clair Patterson's crusade against lead contamination, which resulted from his work in the lab and led to the ban on lead paint and leaded gasoline.
--------------
Yes ! I never knew about that.
 Amy wrote: "Chapter 4
Amy wrote: "Chapter 44) Given the fact that we recently had a "near-miss" with an asteroid, I think I'm reassured by the assertion that "an asteroid passing close to the sun has only slightly better than one chance in a billion of hitting our planet." I THINK. Although the subsequent comment that "we're on one long, deadly carousel ride through the universe" didn't make me feel very safe! ..."
------------
Yes. I thought the same thing. A very timely discussion.
 Amy wrote: "Chapter 4
Amy wrote: "Chapter 45) Love, love, LOVE that the chapter ended with a quote from Carl Sagan. When I was growing up, I was obsessed with Cosmos (both the book and the TV series).
-----------------
Yes, I remember watching that series years ago. I had a hardcover copy of the companion book. A cousin "borrowed" it and I never saw it again. :(
I've since purchased a used copy in PB.
I'm enjoying your comments, Amy.
Books mentioned in this topic
Scott's Last Expedition: The Journals (other topics)Race to the End: Amundsen, Scott, and the Attainment of the South Pole (other topics)
The Lost Photographs of Captain Scott: Unseen Images from the Legendary Antarctic Expedition (other topics)
Scott's Last Expedition, V1 (other topics)
The Devil's Rooming House: The True Story of America's Deadliest Female Serial Killer (other topics)
More...
Authors mentioned in this topic
Gordon R. Williamson (other topics)Edward Samuel Behr (other topics)
Sam Kean (other topics)





Book:
Author:
Sam Kean spent years collecting mercury from broken thermometers as a kid, and now he is a writer in Washington, D.C. His work has appeared in the New York Times Magazine, Mental Floss, Slate, Air & Space/Smithsonian, and New Scientist. In 2009 he was a runner-up for the National Association of Science Writers' Evert Clark/Seth Payne Award for best science writer under the age of thirty. He currently writes for Science and is a 2009-2010 Middlebury Environmental Journalism fellow.
When: The discussion will start on March 1, 2013
Where: The discussion will take place in this thread.
Spoiler Etiquette: The book is divided up into 5 parts. PLEASE put the PART & Chapter # at the top of your post.
Book Detail:
Paperback: 416 pages
Publisher: Back Bay Books; Reprint edition (June 6, 2011)
Language: English
ISBN-10: 0316051632
Kindle: Yes. Available in e-book format
Synopsis: Science magazine reporter Kean views the periodic table as one of the great achievements of humankind, "an anthropological marvel," full of stories about our connection with the physical world. Funny, even chilling tales are associated with each element, and Kean relates many. The title refers to gallium (Ga, 31), which melts at 84ËšF, prompting a practical joke among "chemical cognoscenti": shape gallium into spoons, "serve them with tea, and watch as your guests recoil when their Earl Grey ˜eats™ their utensils." Along with Dmitri Mendeleyev, the father of the periodic table, Kean is in his element as he presents a parade of entertaining anecdotes about scientists (mad and otherwise) while covering such topics as thallium (Tl, 81) poisoning, the invention of the silicon (Si, 14) transistor, and how the ruthenium (Ru, 44) fountain pen point made million for the Parker company. With a constant flow of fun facts bubbling to the surface, Kean writes with wit, flair, and authority in a debut that will delight even general readers. 10 b&w illus.